41 labels on repackaged bulk food for resale must include ingredients
leasehoney.com › 2020/12/29 › sticking-to-theSticking to the Standards: Federal Honey Labeling Requirements Dec 29, 2020 · In Missouri, the honey must include two labels. The first primary/principle display panel is required to have the product’s common name and net quantity (weight in English and metric measurements). If the PDP is the sole label, it must include a list of ingredients by weight in descending order and the name and address of the company. › en › health-canadaIndustry Guide for the labelling of cosmetics - Canada.ca 1.4 Information on labels. This guide covers three aspects of information appearing on the labels of cosmetic products: the classification of cosmetic products (see section 3). required declarations that must appear on a label. These include: product identity (see section 4), net quantity (see section 5),
› pesticide-labels › pesticide-labelingPesticide Labeling Questions & Answers | US EPA Oct 14, 2021 · If labels do not specifically state that they can be used in food storage facilities or food processing plants, can these rodenticides be used under 21 CFR 110.35(c), which states in part " The use of insecticides or rodenticides is permitted only under precautions and restrictions that will protect against the contamination of food, food ...
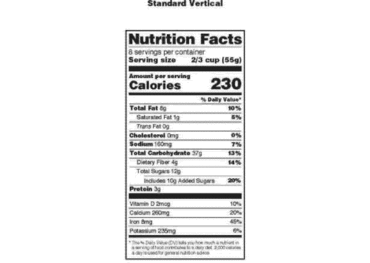
Labels on repackaged bulk food for resale must include ingredients
› en › health-canadaGood manufacturing practices guide for drug products (GUI-0001) About this document 1. Purpose. This guide is for people who work with drugs as: . fabricators; packagers; labellers; testers; distributors; importers; wholesalers; It will help you understand and comply with Part C, Division 2 of the Food and Drug Regulations (the Regulations), which is about good manufacturing practices (GMP). rules.sos.ri.gov › regulations › partPharmacists, Pharmacies, and Manufacturers, Wholesalers, and ... The medication is repackaged in an appropriate USP approved multi-unit, unit-of-use, or single-unit dose container. 4. The pharmacy records the previously repackaged medication quantity, includes all information on the original prescription label, and a pharmacist verifies the medication that was repackaged is correctly labeled by the pharmacy.
Labels on repackaged bulk food for resale must include ingredients. rules.sos.ri.gov › regulations › partPharmacists, Pharmacies, and Manufacturers, Wholesalers, and ... The medication is repackaged in an appropriate USP approved multi-unit, unit-of-use, or single-unit dose container. 4. The pharmacy records the previously repackaged medication quantity, includes all information on the original prescription label, and a pharmacist verifies the medication that was repackaged is correctly labeled by the pharmacy. › en › health-canadaGood manufacturing practices guide for drug products (GUI-0001) About this document 1. Purpose. This guide is for people who work with drugs as: . fabricators; packagers; labellers; testers; distributors; importers; wholesalers; It will help you understand and comply with Part C, Division 2 of the Food and Drug Regulations (the Regulations), which is about good manufacturing practices (GMP).






/cdn.vox-cdn.com/uploads/chorus_asset/file/19328405/566027207.jpg.jpg)







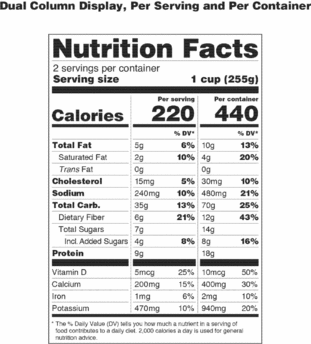













Post a Comment for "41 labels on repackaged bulk food for resale must include ingredients"